Background: Primary central nervous system lymphoma (PCNSL) is a rare form of non-Hodgkin lymphoma with historically poor outcomes. Induction therapy with high-dose methotrexate (HD-MTX)-based regimens followed by consolidation with autologous stem cell transplant (auto-SCT) has become the mainstay of treatment with overall response rates (ORR) of 69-87% and two-year overall survival (OS) of 66-70% reported in prospective clinical trials. Limited data are available regarding a maintenance approach with single-agent HD-MTX, particularly in patients who are not auto-SCT candidates. Herein, we describe a comparison of outcomes in patients with PCNSL who underwent HD-MTX-based induction followed by either HD-MTX maintenance or auto-SCT consolidation at Mayo Clinic, Rochester.
Methods: Patients with a diagnosis of PCNSL who received HD-MTX as part of induction therapy at Mayo Clinic between October 2010 and June 2022 were identified. Patients with prior or concurrent diagnosis of systemic lymphoma were excluded. Primary endpoints were progression-free survival (PFS) defined as time from post-induction treatment initiation to relapse, progression, or death due to any cause; and OS defined as time from post-induction treatment initiation to death due to any cause. We compared baseline characteristics by post-induction therapy using descriptive statistics. The primary endpoints PFS and OS were evaluated using Kaplan-Meier curves and compared by risk scores using a log-rank test.
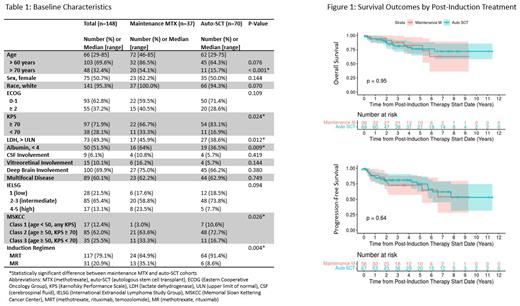
Results: A total of 148 patients were identified (51% female, 95% white) with median age at diagnosis of 66 years (range 29-85), with 48 patients (32%) age > 70. Most patients had multifocal disease (n=89, 60%) and deep brain involvement (n=100, 70%) at diagnosis. Few had vitreoretinal (n=15, 10%) or CSF involvement (n=9, 6%) at diagnosis. Using the Memorial Sloan Kettering (MSKCC) prognostic score for PCNSL, 35 patients (26%) scored class 3 (poor prognosis), 85 (62%) scored class 2, and 17 (12%) scored class 1. In total, 117 patients (79%) received methotrexate, rituximab, and temozolomide (MRT) induction therapy, and the remaining 31 patients (21%) received methotrexate and rituximab (MR) induction therapy (Table 1).
A total of 70 patients (47%) underwent consolidation with auto-SCT and 37 patients (25%) received maintenance methotrexate. The patients who received maintenance methotrexate had higher median age of 72 versus 62 years, higher proportion of patients older than 70 (54% versus 16%, p<0.001), higher proportion of MSKCC prognostic score class 3 patients (33% versus 17%, p=0.026), and trend towards higher proportion of patients ECOG 2 or higher (41% versus 29%, p=0.109). A higher proportion of patients who underwent auto-SCT received MRT (vs MR) induction (n=64, 91%) compared to those who received maintenance methotrexate (n=24, 65%, p=0.004) (Table 1).
The median follow-up for all patients was 4.5 years. At 1-, 3-, and 5-years post-induction treatment initiation, the PFS in the auto-SCT cohort was 85.8% (95% CI 77.7-94.9), 81.0% (95% CI 72.0-91.0), and 74.6% (95% CI 63.3-87.9), respectively; and the PFS in the HD-MTX maintenance cohort was 88.6% (95% CI 78.7-99.8), 72.6% (95% CI 58.8-89.8), and 72.6% (95% CI 58.8-89.8), respectively. At the same time points, the OS in the auto-SCT cohort was 94.0% (95% CI 88.4-99.9), 81.3% (95% CI 71.8-92.0), and 76.0% (95% CI 65.0-89.0), respectively; and the OS in the HD-MTX maintenance cohort was 94.3% (95% CI 86.9-100.0), 87.5% (76.8-99.8%), and 82.4% (69.0-98.4), respectively. Overall, there was no significant difference in PFS (p=0.64) or OS (p=0.95) based on post-induction treatment (Figure 1).
Conclusions: Our study demonstrates comparable outcomes in PCNSL between a post-induction treatment approach with maintenance HD-MTX and consolidation auto-SCT, even though baseline characteristics indicate that patients who went on to receive auto-SCT were younger and had overall better performance status. This suggests that maintenance HD-MTX is a reasonable, time-limited strategy for patients with PCNSL responding to initial induction therapy, particularly in patients who are not candidates for auto-SCT.
Disclosures
Ansell:ADC Therapeutics, Affimed, Bristol-Myers Squibb Company, Pfizer Inc, Regeneron Pharmaceuticals Inc, Seagen Inc, Takeda Pharmaceuticals USA Inc.: Other: Contracted Research. Habermann:BMS: Research Funding; sorrento: Research Funding; Genentech: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal